How Reagent Purity Impacts API Manufacturing for Oligonucleotides and Peptides
The therapeutic potential of oligonucleotides and peptides is reshaping the landscape of modern medicine. These complex molecules, often referred to collectively as TIDES (Therapeutic Oligonucleotides and Peptides), represent a significant advancement in drug development. Offering highly targeted interventions for a range of challenging diseases, from rare genetic disorders to metabolic conditions and cancers. As active pharmaceutical ingredients (APIs), their mechanisms—whether modulating gene expression or mimicking natural biological ligands—hold immense promise.
However, translating this promise into effective therapies depends on robust and efficient API manufacturing. The synthesis of these large, sequence-specific biopolymers is inherently complex, involving multiple, repetitive chemical steps where precision is paramount. Among various contributors to success or failure, purity of chemical reagents and pharmaceutical raw materials stand out as a critical factors.
The Growing Complexity of API Manufacturing
As demand for oligonucleotide and peptide therapeutics continues to grow, manufacturers face increasing molecular complexity: longer sequences and more unique modifications.
Inefficiencies or impurities in a single step can cascade across dozens of cycles—magnifying errors, reducing yield, and complicating purification. Reagent purity becomes an essential factor in quality and achieving lower Process Mass Intensity (PMI).
Understanding the Role of Reagents in TIDES API Synthesis
API manufacturing for oligonucleotides and peptides utilizes stepwise chemical synthesis, where consistency and activity of each reagent directly affects cycle efficiency. Impurities introduced during production can compromise downstream API workflows.
Oligonucleotide Reagents and Process Requirements
Oligonucleotide synthesis typically uses the phosphoramidite method via solid-phase synthesis (SPS), relying on a four-step cycle: detritylation, coupling, oxidation/sulfurization, and capping. Critical reagents include phosphoramidite building blocks, activators, oxidizing/sulfurizing agents, and anhydrous solvents like acetonitrile.
Peptide Reagents and Workflow Design
Peptide synthesis mainly happens through Solid Phase Peptide Synthesis (SPPS). Liquid Phase Peptide Synthesis (LPPS) approaches are also used, especially in hybrid workflows. SPPS uses cycles of deprotection and coupling with key reagents including protected amino acids, coupling reagents, bases, and solvents.
In both synthesis types, impurity-laden reagents can interrupt the chemistry, introducing deletion sequences, promoting side reactions, or triggering failed couplings. Ensuring upstream control during reagent synthesis is vital to mitigating these risks and maintaining downstream process integrity.
Where Reagent Impurities Affect TIDES Synthesis Most
Understanding exactly where these vulnerabilities lie is essential to managing risk. Each stage of the synthetic cycle, whether it is oligonucleotide or peptide-based, has its own sensitivity to impurities. Many of these impurities result from inconsistencies in the production of the reagents.
The Effect of Poor Reagent Control
These issues are not just technical problems. They cause uncertainty in processes, raise the risk of batch rejections, and make purification harder. As molecule complexity rises and regulatory expectations tighten, the downsides of relying on substandard inputs become more pronounced, especially in high-volume API manufacturing. Examples include:
- Water Content: Residual moisture can hydrolyze intermediates, reduce coupling efficiency, and truncate sequences. Anhydrous reagents are critical.
- Oxidation/Sulfurization Issues: Incomplete or incorrect conversion affects backbone structure.
- Incomplete Coupling: Poor-quality reagents reduce efficiency, creating deletion sequences.
- Side Chain Reactions: Impurities can catalyze oxidation or alkylation.
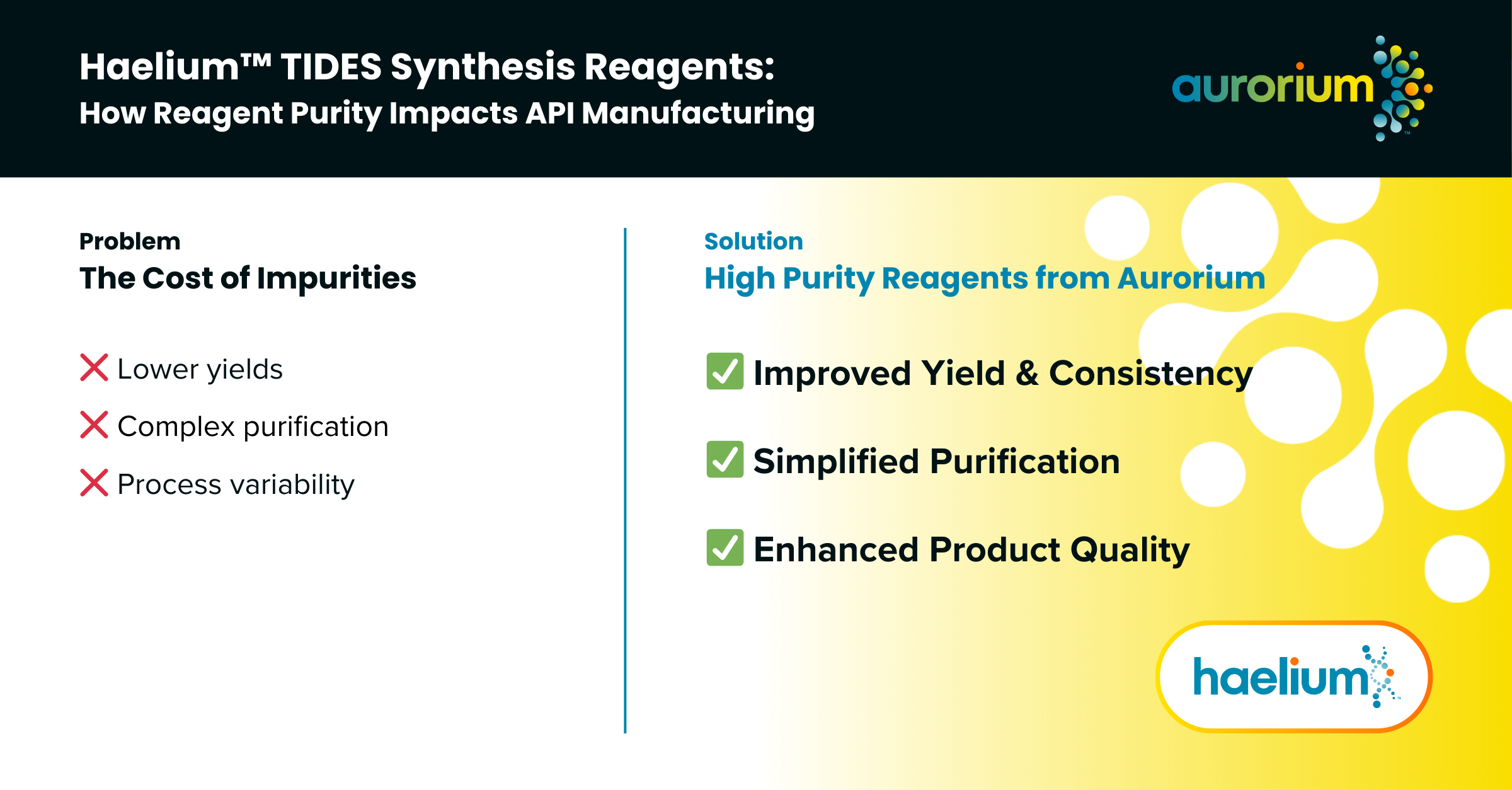
The Cost of Impurities
Beyond the lab, the consequences of impurity-related inefficiencies extend deeply into operational and commercial outcomes. In API manufacturing, even small contaminants in pharmaceutical raw materials can cause problems. They can impact product quality, plant efficiency, supply timelines, and regulatory readiness.
Challenges of Impurity Management
These challenges show how reagent variability ripples through the entire value chain:
- Lower yields increase cost of goods sold (COGS) due to higher waste and reprocessing.
- Complex purification demands more solvents, equipment time, and labor — all of which reduce batch throughput.
- Process variability from inconsistent reagents leads to unpredictable timelines, more QA interventions, and batch release delays.
For TIDES manufacturers, variability in raw material quality doesn’t just increase operational complexity — it risks failed batches and regulatory challenges. These can delay timelines and impact revenue.
When multiplied across commercial-scale production, these issues delay timelines, strain supply chains, and hurt profitability.
Reagent Purity as an Enabler – Not a Bottleneck
For manufacturers working at scale, using high purity reagents and controlled pharmaceutical raw materials is crucial. This helps improve product quality and operational efficiency. These materials are not just inputs — they are key enablers of reduced cycle variability, regulatory compliance, and ultimately, successful API manufacturing outcomes. Across both development and commercial stages, they help ensure reactivity, reliability, and control throughout the synthesis process.
Strategic Advantages of High Purity Reagents
- Improved Yield and Consistency: Better inputs produce more reliable outcomes.
- Simplified Purification: Fewer byproducts reduce downstream burden.
- Enhanced Product Quality: Easier compliance with purity specs.
- Cost Savings: Reduced rework, lower waste, faster cycle times.
- Regulatory Alignment: Easier GMP validation with consistent inputs.
To boost production for clinical or commercial use, peptide synthesis companies should partner with a chemistry reagents manufacturer. This manufacturer should understand the specific risks involved and provide consistent reagent synthesis and documentation. This partnership is key to achieving success at a larger scale.
Haelium TIDES Reagent Synthesis Case Studies
Reducing Primary Amines in LPPS
Haelium™ Piperidine 800, formulated to reduce primary amine content by 10x compared to standard piperidine grades. Results showed that side reactions during piperidine deprotection in LPPS could decrease by 0.4% per synthesis cycle. Directly improving crude peptide API purity and reducing process mass intensity (PMI).
Maximizing Sulfurization Efficiency in Oligo Synthesis
Trace water in solvents like pyridine and β-picoline degraded water-sensitive moieties. Haelium™ Pyridine 900, which contains 30x lower water and reactive impurity levels, helped reduce sulfurization reagent degradation by up to 10% per cycle ensuring >99.9% sulfurization efficiency.
Unlocking the Full Potential of MsPA Linkers
To support the development of modified oligonucleotides, Haelium™ Mesyl Chloride was engineered to reduce chloromesyl chloride levels by 50x. Achieving >99.99% assay purity. This enabled broader use of MsPA linkers in oligonucleotide design, improving therapeutic stability and delivery performance.
Why It’s Time to Rethink Reagents in API Manufacturing
As the pharmaceutical industry embraces more complex peptide and oligonucleotide therapies, the need for high-purity, well-characterized reagents becomes central to scalable success.
Achieving efficiency and consistency in large-scale oligonucleotide synthesis demands high purity reagents with ultra-low moisture content and tightly specified impurity profiles. Especially for critical steps like coupling and oxidation.
Aurorium’s Approach to Reagent Innovation
Aurorium designed its Haelium™ Reagent Solutions specifically to address these evolving needs in modern API manufacturing. With offerings including:
- Haelium™ Piperidine 800: where primary amines are reduced by 10x compared to standard piperidine.
- Haelium™ Pyridine 900: Ultra-low moisture (<30 ppm) for oxidation/sulfurization steps.
- Haelium™ Lutidine 500: Reliable performance and color stability in oligonucleotide workflows.
- Haelium™ AccelOx 9010: Efficient oxidation to improve synthesis speed and throughput.
Backed by cGMP North American manufacturing, technical chemistry expertise, and a partnership mindset. Aurorium and its Haelium product range offer a path to more reliable, efficient, and scalable TIDES manufacturing.
To ensure the quality and consistency required for your critical oligonucleotide and peptide synthesis processes, explore solutions for sourcing rigorously tested, high purity reagents.
Contact an Aurorium expert today to discuss how Haelium™ high purity reagents and pharmaceutical raw materials can support your specific API manufacturing needs.
